ASTMH 2017 Poster - The potential for six-monthly mass administration of moxidectin to accelerate onchocerciasis elimination
This is adapted from a poster presented at ASTMH 2017 by P. Milton, M. Walker, A.C. Kuesel, N.O. Opoku, S.K. Attah, E. Kanza, D. Bakajika, H. Howard, C.M. Halleux, C.R. Rayner, D. Smith, G. Pearce, M. Sullivan, M.G. Basáñez
Introduction
African onchocerciasis control and elimination programmes rely predominantly on annual community-directed treatment with ivermectin (aCDTI). However, modelling results indicate that aCDTI may not be sufficient to reach the current goals of eliminating onchocerciasis in 80% of endemic African countries by 2025.
Phase II and III clinical trials have shown that moxidectin, a veterinary anthelmintic, is a more efficacious treatment, suppressing skin microfilarial loads for longer. This study assessed the potential impact of community-directed treatment with moxidectin given annually (aCDTM) or biannually (bCDTM) compared to increasing CDTI to biannual (bCDTI).
Methods
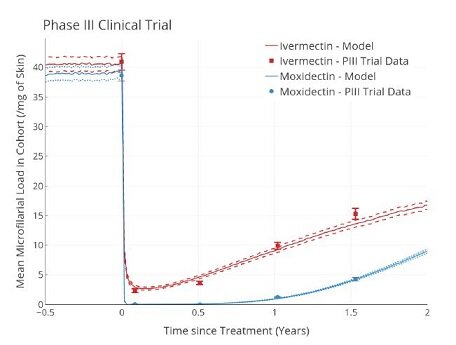
Using clinical trial data and our individual based, stochastic transmission model EPIONCHO-IBM, we capture skin microfilarial (mf) dynamics in response to
treatment with ivermectin and moxidectin.
Skin mf dynamics for ivermectin (red) and moxidectin (blue) matching clinical trial:
- Baseline endemicity
- Inclusion/exclusion criteria
- Pregnancy status
Moxidectin Dose
Phase II trial data 1 showed that lower moxidectin doses (2mg, 4mg) have shorter microfilarial suppression than 8mg moxidectin.
In line with the reduced trial efficacy, the probability of eliminating the parasite was greatest for 8mg moxidectin, regardless of:
- Programme duration (15, 20, 25y)
- Endemicity (40-60% prevalence)
- Treatment coverage (65% or 80%)
Elimination probability when using 2mg (blue) or 4mg (red) moxidectin administered annually (no fill) or biannually (filled).
Rates are relative to the equivalent scenarios using an 8mg dose (horizontal dashed line). (Values <1 indicate a lower ability to eliminate.)

Health Economics
Biannual distribution of ivermectin in Ghana was shown to have an approximate 50–60% increase in cost compared to aCDTI 3. Thus, despite faster times to
elimination, bCDTI and bCDTM incur increased costs of elimination compared to aCDTI.
Annual moxidectin does not have the added costs of biannual distribution and is thus more cost-effective for achieving elimination (assuming donation of
moxidectin).
The economic costs required to achieve elimination relative to aCDTI with ivermectin (blue) or moxidectin (red) given annually (no fill, aCDTM) or biannually
(filled, bCDTM). (Values <1 indicate that the cost of elimination is less than aCDTI).
Elimination is defined as having 95% of runs showing no infection (in humans and vectors) 50 years after treatment cessation.
Conclusions
Moxidectin would be superior to ivermectin for the treatment and elimination of onchocerciasis via MDA, achieving faster times to elimination for a given
treatment frequency and coverage.
The increased cost incurred with biannual distribution indicates that annual moxidectin would be more economically viable than the currently proposed
alternative treatment strategy of switching from aCDTI to bCDTI (assuming that moxidectin would be donated).
To achieve the fastest possible elimination or for areas of very high pre-treatment endemicity, bCDTM could be used and remains more economical than bCDTI.
(slowest) aCDTI > bCDTI ≈ aCDTM > bCDTM (fastest)
References
- Awadzi et al. (2014) A randomized, single-ascending-dose, ivermectin-controlled, double-blind study of moxidectin in Onchocerca volvulus infection. PLoS Negl Trop Dis 2014, 7(6): e2953
- Turner HC, Walker M, Churcher TS, Basáñez MG (2014) Modelling the impact of ivermectin on River Blindness and its burden of morbidity and mortality in African Savannah: EpiOncho projections Parasites & Vectors 2014, 7:241
- Turner HC, Osei-Atweneboana MY, Walker M, Tettevi EJ, Churcher TS, Asiedu O, Biritwum NK, Basáñez MG.(2013) The cost of annual versus biannual community-directed treatment of onchocerciasis with ivermectin: Ghana as a case study. PLoS Negl Trop Dis 2013 7(9): e2452
Acknowledgements & Funding
The authors thank Medicines Development for Global Health for providing both the Phase II and Phase III trial data and the many parasitologists who collected the data in the field. This work was funded by MDGH.